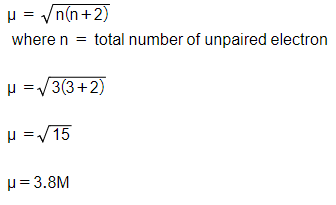No links please Calculate spin only magnetic moment of Fe3+ ion - Chemistry - Alcohols Phenols and Ethers - 12302421 | Meritnation.com
What will be the theoretical value of spin only magnetic field when Fe(SCN)3 reacts with the solution containing F ions to yield a complex
![The complex with spin only magnetic moment of ~4.9 BM is:a)[Fe(H2O)6]2+b)[Fe(CN)6]3-c)[Fe(CN)6]4-d)[Fe(H2O)6]3+Correct answer is option 'A'. Can you explain this answer? - EduRev Chemistry Question The complex with spin only magnetic moment of ~4.9 BM is:a)[Fe(H2O)6]2+b)[Fe(CN)6]3-c)[Fe(CN)6]4-d)[Fe(H2O)6]3+Correct answer is option 'A'. Can you explain this answer? - EduRev Chemistry Question](https://edurev.gumlet.io/ApplicationImages/Temp/9569037_ab072f64-2e6d-4920-b550-ecfc86b0a8de_lg.png)
The complex with spin only magnetic moment of ~4.9 BM is:a)[Fe(H2O)6]2+b)[Fe(CN)6]3-c)[Fe(CN)6]4-d)[Fe(H2O)6]3+Correct answer is option 'A'. Can you explain this answer? - EduRev Chemistry Question
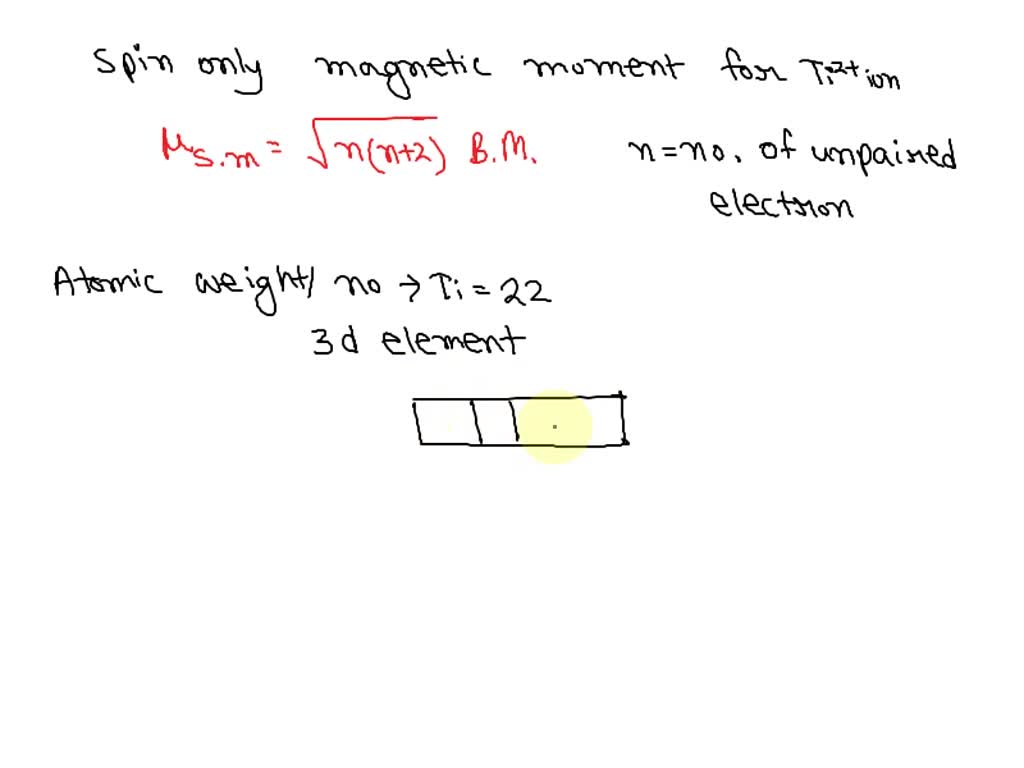
SOLVED: 'The calculated spin only magnetic moment for Ti2+ metal ion is a 3.87 b 1,.73 C. 4.92 d. 2.83'

The highest value of the calculated spin-only magnetic moment (in BM) among all the transition - YouTube
The effective magnetic moment of hexaammonianickel+2 is 3.20 BM. Is it higher, lower, or equal to the Miu spin only value? - Quora

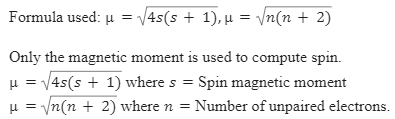

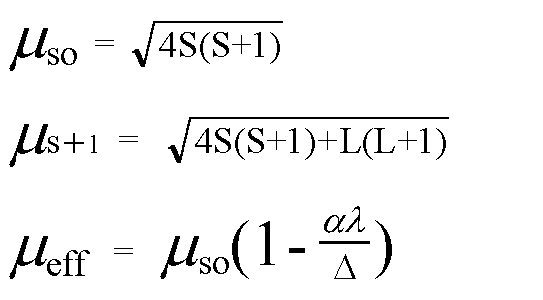

![Spin only magnetic moment of the compound Hg [C0 (SCN)(4)] is Spin only magnetic moment of the compound Hg [C0 (SCN)(4)] is](https://static.doubtnut.com/ss/web/416446.webp)